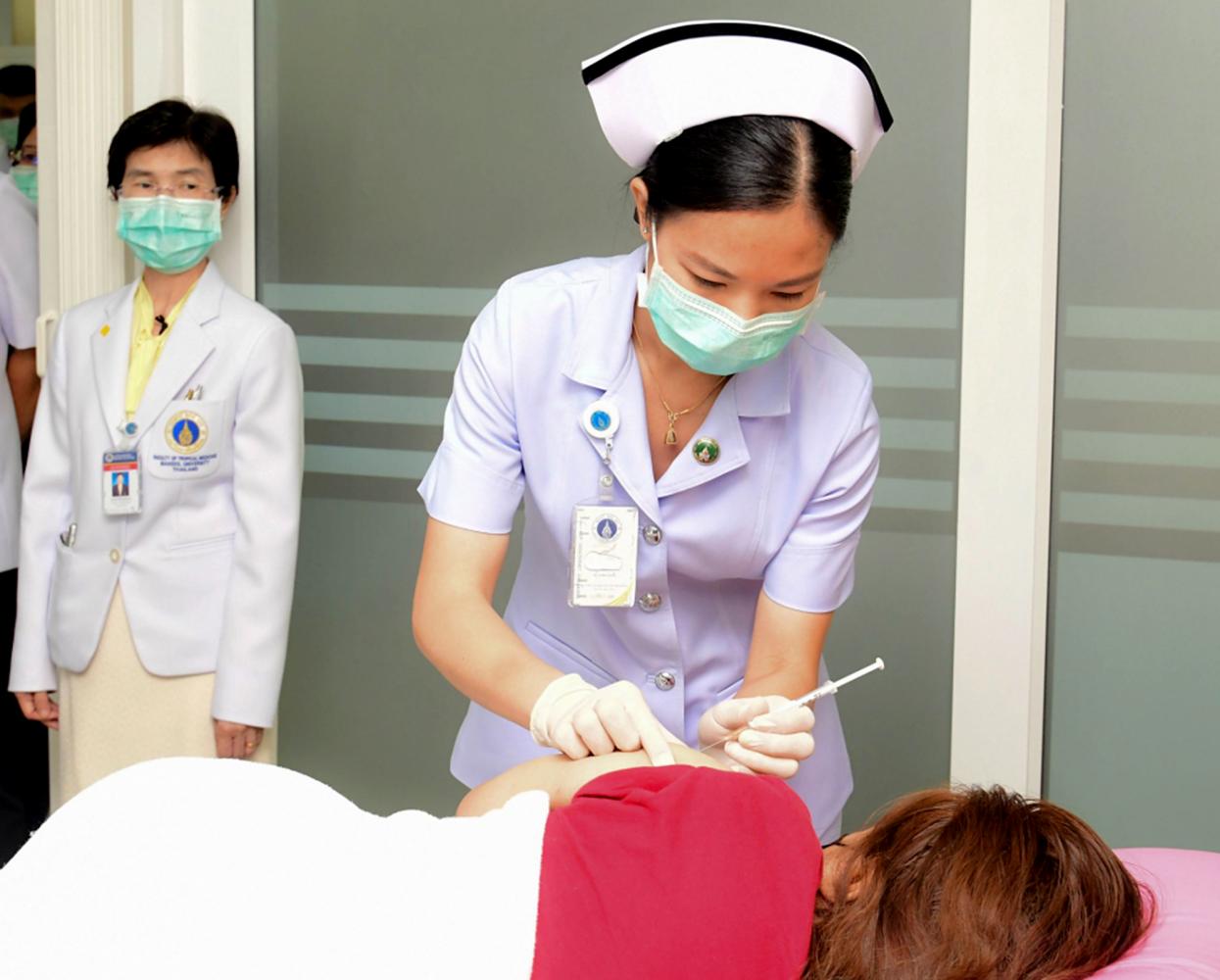
Thailand on Monday started the first human trials of a domestically developed Covid-19 vaccine, with the rollout expected to begin next year.
The vaccine, consisting of an inactivated virus to trigger immunity, is being developed by the Government Pharmaceutical Organisation (GPO) using egg-based technology.
The GPO has been working on the project with Mahidol University's Tropical Medicine Department, the Programme for Appropriate Technology in Health (PATH), an American non-profit organisation, and the Icahn School of Medicine at Mount Sinai, New York. Together they have developed a pilot NDV-HXP-S vaccine and lab tests have been showing promising results.
Public Health Minister Anutin Charnvirakul said the development of the home-grown vaccine is a very significant step for the country's public health, as it would enable Thailand to be self-reliant and it would not need to depend on vaccines from foreign countries.
The information from the human trials will be used to apply for registration with the Food and Drug Administration (FDA) for industrial production at the GPO's vaccine production plant in Saraburi's Kaeng Khoi district.
The plant already has egg-based technology to make flu vaccines, which will be adjusted to produce Covid-19 vaccines, he said.
"Production is expected to begin next year, with an estimated 25-30 million doses annually," Mr Anutin said.
The minister was confident that the success of home-grown vaccine production would help Thailand to manage its vaccination strategy more effectively.
"Even though we can produce vaccines in the country, it is from technology transfer and under management of the owners of the brands and technology," he told the news conference.
"But today, if we are successful we can be self-reliant and determine our own direction."
Banchong Mahaisavariya, president of Mahidol University, said the first and second phases of human trials will be to test the safety of the vaccine and its ability to trigger immunity.
A total of 460 volunteers would be recruited for the human trials, 210 of whom are aged 18-59 and would take part in the first phase. They must be in good health and free of Covid-19. Volunteers will be screened and have their backgrounds checked and undergo health and blood checks.
Of the 210 volunteers, the first group of 18 would be given the smallest amount of vaccine which would be increased gradually while testing would be conducted on the other 192 volunteers, Dr Banchong said.
Phase two is expected to begin in July, with testing on 250 volunteers aged between 18-75. Results are expected to be known by the end of the year, he said.
Dr Punnee Pitisuttithum, head of the Vaccine Trial Centre at Mahidol University's Tropical Medicine Department, said that the first human trial was conducted on four volunteers on Monday.
After 30 minutes of receiving the vaccine, they were observed for another four hours before being allowed to go home, Dr Punnee said.
Withoon Danwiboon, managing director of the GPO, said on Monday that if the first and second phases of the human trials are successful, the third-phase trial would need more volunteers, which may include people in other countries.
"For maximum safety, we may have to conduct the mass testing on people in foreign countries with larger numbers of infections than Thailand. If the procedure is completed, we can produce the vaccine next year," he said.
He also said GPO was aware of SARS-CoV-2 virus mutations so the vaccine is also being developed to help with this situation, adding that lab tests found the pilot vaccine showed it was effective against the South African strain.
Another homegrown vaccine is being developed by Chulalongkorn University and uses Messenger RNA (mRNA) technology. Human trials are expected to begin soon.
